Biologics quality testing
Faster, safer, animal free.
iDTECT® assays by PathoQuest provide a unique NGS Quality Control platform for biologics.
Accepted by Industry and Regulators.
Why PARTNER WITH PathoQuest?
At PathoQuest, we understand the critical need to develop your innovative biologics safely and rapidly in a complex biosafety testing environment. PathoQuest provides a validated Next Generation Sequencing (NGS) approach in biosafety testing, biologics characterization, and biologics release for global biopharmaceutical and emerging biologics companies.
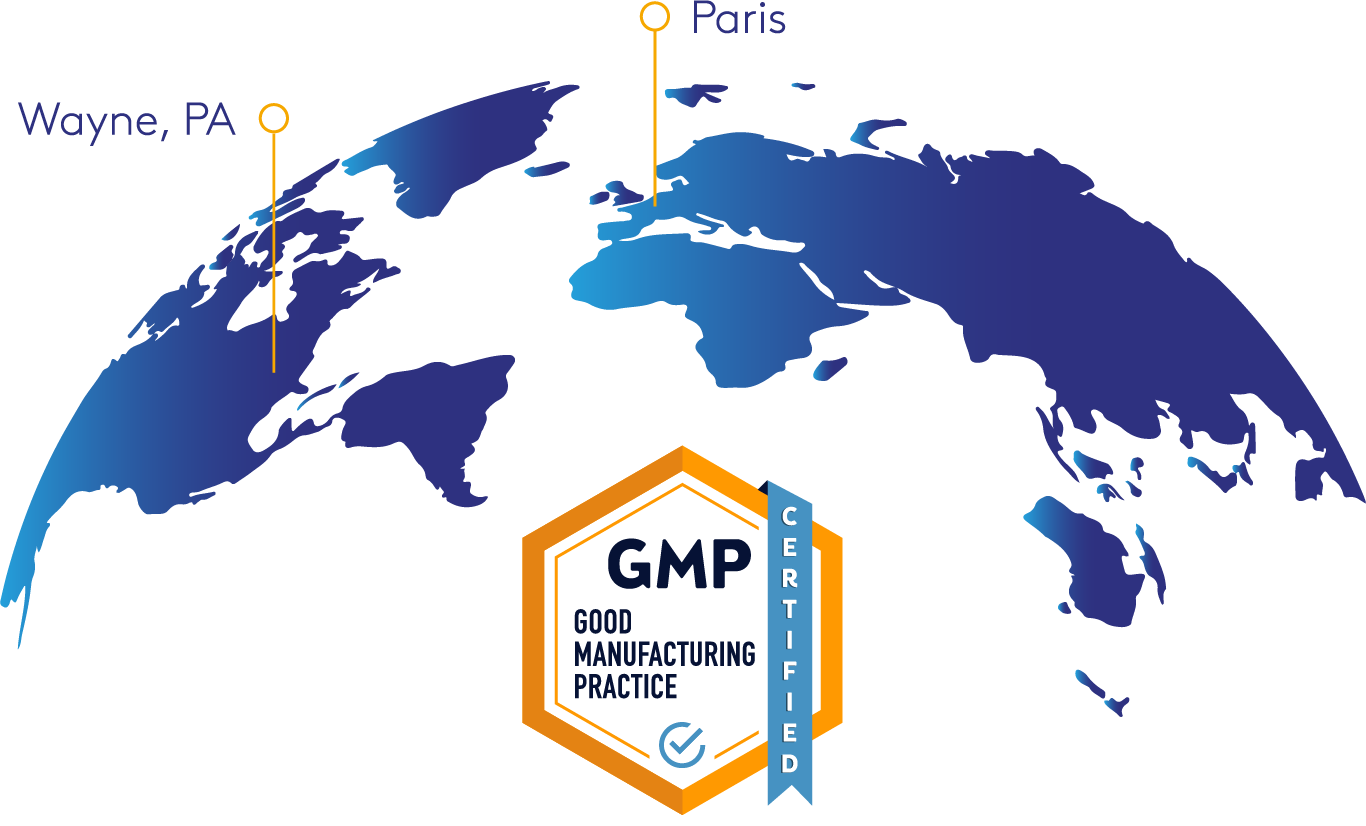
With a proven track record in biotesting, PathoQuest offers a team of NGS Quality Control (QC) testing experts based in France and the U.S. Our expert Contract Research Organization (CRO) team is readily available to support with rapid and robust QC testing at both GMP and non-GMP levels, emphasizing our commitment to biosafety services.
Let’s work together to transform the future of biologic therapies and make a significant impact on patient lives.
OUR UNIQUE NGS QUALITY CONTROL ASSAYS
Discover iDTECT® platform
Our brand for industry-leading NGS bioassays
For viral safety and genetic characterization
GMP Quality Control
OUR HAPPY CUSTOMERS
Modalities
mAbs and Recombinant Proteins
Bacterial and mammalian produced proteins, hormones and peptides
READ MORE
Viral Vectors
Viral gene delivery, oncolytic and immunotherapy including manufacturing plasmids
READ MORE
Cell Therapies
Including gene modified, or unmodified stem cell therapies, allogeneic or autologous
READ MORE
Vaccines
Inactivated, live-attenuated, recombinant, RNA and viral vector products
READ MORE
RNA
Immunotherapies, antiviral, vaccines, RNAi and CRISPR based gene editing
READ MORE
Cultivated Meat
Engineered cells and tissues cultured in more ethical in vitro environments
READ MORE
3Rs
Next Generation Sequencing (NGS) is the ethical alternative to in vivo testing for biologics testing.
PathoQuest, in partnership with Charles River Laboratories, pioneers in demonstrating the superiority of NGS-based biosafety testing over traditional biosafety testing and biologics characterization. Our advanced biotesting services set new standards, replacing conventional methods with faster, safer, and animal-free alternatives in biologics testing.
3Rs
Next generation sequencing is the ethical alternative to in vivo testing for the Quality Control testing of biologics.
PathoQuest, in partnership with Charles River Laboratories, is the first CRO to conclusively demonstrate the superiority of NGS over conventional in vivo biosafety testing and characterization.

SERVICES
Adventitious Virus Testing
Detection of viral contamination within the manufacturing process and beyond.
Identity Confirmation
Genetic characterization of viral and plasmid products for release.
Integration Site Analysis
Characterisation of genetic modifications for clone selection, genetic stability and lot release
Cell Line Characterization
Biosafety screening and stability testing of manufacturing cells.
HLA Genotyping
Characterizing and screening for novel and emerging cell therapies.
In Vivo Replacement
NGS as an ethical alternative to animals in biosafety testing and characterization.
Raw Material Testing
Screening of high-risk inputs such as animal products and media.
READ MORE
READ MORE
READ MORE
SERVICES
Adventitious Virus Testing
Detection of viral contamination within the manufacturing process and beyond.
READ MORE
Identity Confirmation
Genetic characterization of viral and plasmid products for release.
READ MORE
Cell Line Characterization
Biosafety screening and stability testing of manufacturing cells.
READ MORE
HLA Genotyping
Characterizing and screening for novel and emerging cell therapies.
READ MORE
In Vivo Replacement
NGS as an ethical alternative to animals in biosafety testing and characterization.
READ MORE
Raw Material Testing
Screening of high-risk inputs such as animal products and media.
READ MORE
STRATEGIC PARTNERSHIPS
MEMBERSHIPS
News and Events
Contact us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
Sign up for our latest news